Get full access with a free account
Benefits of the Coloplast® Professional Educational platform
![]() Full access to educational content, events and resources
Full access to educational content, events and resources
![]() Track your progress
Track your progress
![]() Share content with your colleagues
Share content with your colleagues
![]() Share supporting material with your patient
Share supporting material with your patient

Infection and biofilm in Wound & Skin Care
A wound is considered infected when microorganisms that damage local tissue and delay wound healing are present.
In this section
In this section, we'll cover off the following areas:
Managing risk of infection
24% of HCPs believe that risk of infection is a challenge when treating wounds. 1
To minimise the risk of infection and simplify practice, it is key to have a wound bed conforming dressing that absorbs and retains the exudate and bacteria.
This section should help you to better understand wound infection, causes of infection, the role of microorganisms, the stages of infection and how to tell if a wound is infected.
Understanding Infection
What is wound infection?
A wound is considered infected when microorganisms that damage local tissue and delay wound healing are present.1
The presence of microorganisms triggers an immune response in the patient. Whether or not this response is successful depends on a balance of two things:
- the strength of the patient’s immune system; and
- the amount and virulence of the pathogens.
What causes wound infection?
When the amount and virulence of the pathogens are too much for the patient’s immune system to handle, the wound becomes infected.2
What role do microorganisms play in a wound?
It’s important to note that all wounds contain microorganisms, and their presence does not necessarily mean the wound is infected. Keep in mind that:
- a wound’s bacterial status can change depending on local, environmental and systemic factors; and
- the transition from non-infected to infected wounds is often gradual.
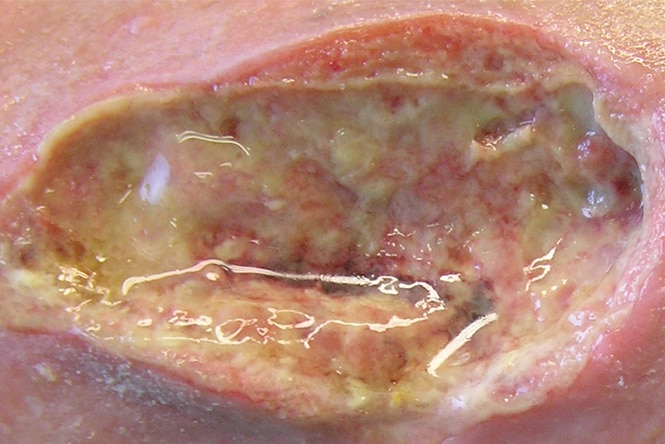
What are the five stages of wound infection?
A wound’s microbial balance can be described as a continuum.1 There is a gradual increase in the number and virulence of microorganisms. As this occurs, the patient’s immune system responds accordingly.
The figure below shows the five stages of wound infection:
- Contamination
- Colonisation
- Local infection
- Spreading infection
- Systemic infection
How can you tell a wound is infected?
It can be challenging to identify infection in chronic wounds. By performing a holistic wound assessment, you can evaluate the patient’s risk of infection. That will allow you to proactively manage the wound to reduce the risk of infection.3
If you don’t have access to modern microscopy tests to identify the organism causing the infection, you can use the following approach:
- Check for clinical signs and symptoms.
- Take a wound culture to identify the causative organisms and their potential resistance to antibiotics.3
What are the clinical signs of wound infection?
In the chart, you’ll see a list of the clinical signs and symptoms of local and systemic infections that you need to look out for when assessing the wound.2
| Signs of local infection | Signs of systemic infection |
|---|---|
|
|
How do you treat an infection?
If you suspect an infection, your immediate goal is to reduce the bioburden in the wound.3 You can do this by:
- therapeutic cleansing of the wound at each dressing change;
- aggressive debriding the surface substance and underlying non-viable or unhealthy tissue. This will disrupt the microbial burden and prevent biofilmfrom reoccurring;3 and
- monitor the wound’s progress and reassess it continually to see if the wound is meeting your treatment goals. You should do this at least once a week or, optimally, at each dressing change.4
Treating specific types of infection
If there are signs of local wound infection, you can use topical antimicrobials. If the infection is spreading beyond the wound area, you will need to use systemic antimicrobials.
If there are signs of a systemic infection, consult a physician or wound specialist immediately.
Biofilm and its role in wound infection
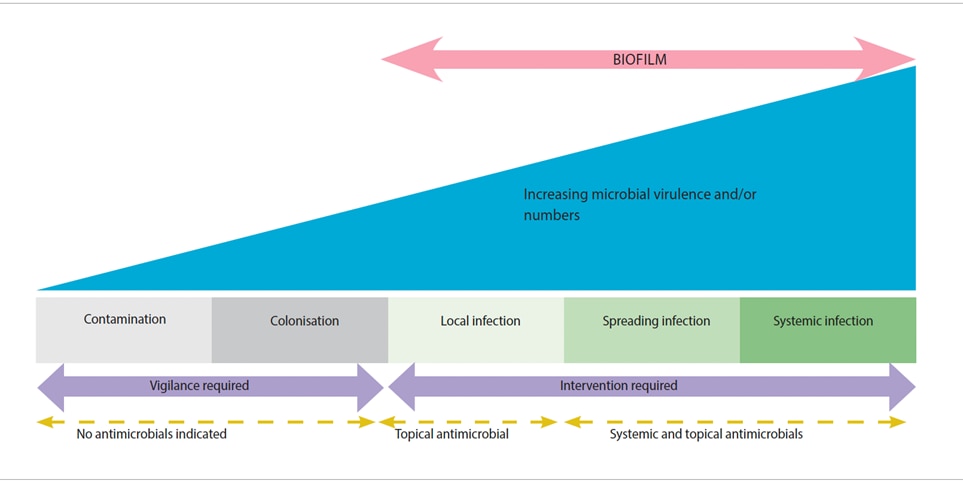
How do biofilm affect wound healing?
Biofilm are probably the most important single cause of persistent, delayed healing in wounds.2 As figure 1 illustrates, they are also thought to delay wound healing by effecting an inappropriate inflammatory response, which is ineffective, poorly orchestrated and damaging to host tissues.2
There is increasing evidence that biofilm are present in most, if not all, chronic, non-healing wounds.3 Therefore, if you diagnose that your patient has an infection in a chronic wound, it’s recommended that you follow the guidelines for preventing and managing biofilm.2
Two types of bacteria formations
Bacteria are often viewed as being single cells that multiply rapidly when in exponential growth. This is referred to as ‘planktonic form’ and relates mostly to acute infections. Bacteria can also form aggregates, or communities, of slow-growing cells – this kind of formation is referred to as ‘biofilm’. The most common biofilm formers are Staphylococcus aureus and Pseudomonas aeruginosa.2
All about Biofilm
How do you detect biofilm?
Detecting biofilm can be challenging, due to the following factors:
- Biofilm are microscopic structures, which are invisible to the naked eye. So you need to use a high-powered microscope to detect them.
- In a clinical setting, the best detection method is a tissue biopsy. However, biofilm are small and unequally distributed in the wound bed. A wound may have different species of biofilm, and they are typically scattered around in small, isolated, single-species islands. That’s why they can be easy to miss.
What are the signs of wound infection?
Given the expense and time involved in tissue biopsy and microscopy, most clinician diagnose biofilm by identifying the common signs of wound infection. Some of these signs are2,4:
- sloughy tissue
- increased levels of exudate
- poor granulation / friable hyper granulation
- malodour
- delayed healing
If a wound still shows these signs of infection after it has been managed correctly and the patient has received appropriate health support, biofilm may be present.
When should you suspect biofilm?
Try asking yourself the following questions2:
- Have all appropriate diagnostic and therapeutic measures been followed?
- Is the wound failing to heal as expected?
- Does the wound show signs of local infection or inflammation?
If you answer ‘Yes’ to at least 2 of these questions, it would be clinically relevant to treat for biofilm.
Example of wound biofilm
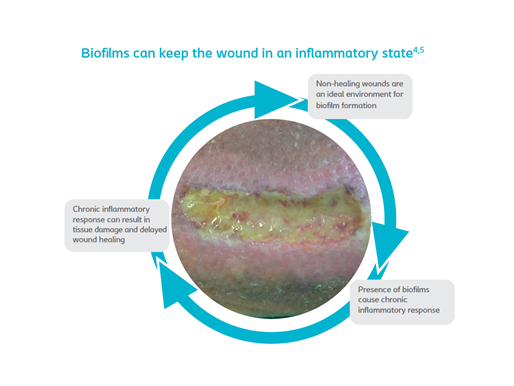
Confocal laser scanning microscopy (CLSM)
Image 1 shows a microscopic image of biofilm (highlighted in red), with clusters often less than 1/10 mm. This results in many swabs coming back inconclusive.
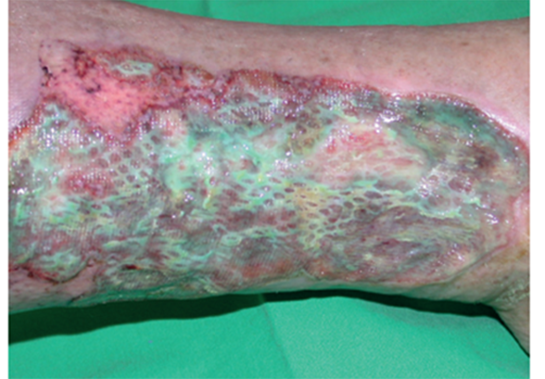
Example of wound with suspected biofilm
You should suspect biofilm in ‘healable’ wounds, that are non-healing, if you have taken all of the appropriate measures.1
Simplifying the treatment of infection and biofilm

Bite sized learning
Covering:
- The Wound Infection continuum
- Biofilms and their effect on wound healing
- Bacteria causing infection
- Infection diagnosis
- How to manage the infected wound
Wound Infection Pathway
Using the Coloplast 3 Step Approach, we've broken down the pathway for Assessing, Preparing and then treating wound infection into a simplified pathway.

Simplifying infection Webinar
Transforming wound care: Simplifying infection management Pathways
28th May 2020
Discover Biatain® Silicone Ag with 3DFit Technology
Biatain Silicone is a conforming dressing with 3DFit® Technology that fills the gap and reduces exudate pooling to promote optimal healing conditions.
References
View References
- International Wound Infection Institute (IWII) Wound infection in clinical practice. Wounds International 2016.
- Keast, David & Swanson, Terry. White paper – A practical summary for the management of wound infections and biofilm, Coloplast 2020
- Swanson, Terry. Keast, David. Bain, Kimberly & Mark. Preventing and treating infection in wounds: translating evidence and recommendations into practice. Wounds International 2020. Vol 11, issue 4
- Dowsett et al. (2020). Closing the gap between the evidence and clinical practice – a consensus report on exudate management (11(3))
